At the end of 2019, an infectious pneumonia (COVID-19) caused by the novel coronavirus (SARS-CoV-2) began to spread around the world.
Regardless of the number of infected people or the spatial extent of the epidemic area, COVID-19 has overwhelmingly exceeded the SARS and MERS epidemics. The continued outbreak of the COVID-19 epidemic poses a serious threat to global public health.
On October 6, 2020, Shi Zhengli’s team published a review titled “Characteristics of SARS-CoV-2 and COVID-19” in Nature Reviews Microbiology. The article summarizes the virological characteristics of the COVID-19 pathogen SARS-Cov-2 in high detail, and compares the differences between SARS-CoV-2 and known human viruses. In addition, this article summarizes the currently known clinical, epidemiological and pathological features of COVID-19, as well as the current research progress in animal models and treatment methods of SARS-CoV-2 infection.

- Characteristics, Receptor Binding and Infection Mechanism of SARS-CoV-2
Coronaviruses are divided into four categories, namely: Alpha-, Beta-, Gamma-, Delta- viruses, among which Beta viruses include MERS-CoV, SARS-CoV and SARS-CoV-2, the pathogen of COVID-2019. As a new type of β-coronavirus, SARS-CoV-2 shares 79% genome sequence similarity with SARS-CoV. The six functional open reading frames (ORFs) are arranged from 5′ to 3′: replicase (ORF1a/ORF1b), Spike (S), envelope (E), membrane (M) and nucleocapsid (N). In addition, the attachment proteins encoded by seven recognized ORFs are interspersed among structural genes.
Phylogenetic analysis of the entire genome showed that SARS-CoV-2, together with the SARS-CoV and SARS-related coronaviruses (SARSr-CoVs) found in bats, belong to the subgenus Sarbecovirus of β-coronavirus. In order to assess the genetic variation of different SARS-CoV-2 strains, the China National Center for Bioinformation (CNCB) has compared the global SARS-CoV-2 genome sequences. A total of 15018 mutations were identified, including 14,824 single nucleotide polymorphisms (BIGD).
A special genomic feature of SARS-CoV-2 is the insertion of four amino acid residues (PRRA) at the junction of the S1 and S2 subunits of the S protein. This insertion can generate multiple alkaline cleavage sites (RRAR), which are effectively cleaved by the paired basic amino acid protease (Furin) and other proteases. A structural study showed that the Furin cleavage site can reduce the stability of the SARS-CoV-2 S protein, which is conducive to the binding of receptor-binding domain (RBD) to the receptor. Compared with SARS-CoV, whether SARS-CoV-2’s higher transmission ability is related to the acquisition of Furin cleavage sites remains to be confirmed.
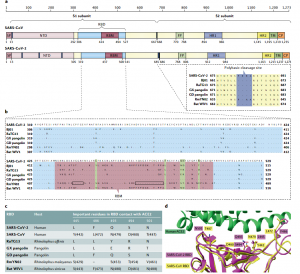
Fig. 1 Key differences in the spike protein of SARS-CoV-2 and related coronaviruses.
SARS-CoV-2 and SARS-CoV have the same receptor, angiotensin-converting enzyme 2 (ACE2). In addition to human ACE2 (hACE2), SARS-CoV-2 can also recognize ACE2 from pigs, ferrets, rhesus monkeys, civet cats, and pangolins. The widespread existence of SARS-CoV-2 receptors in multiple species means that it may have a wide host range. The difference in the efficiency of ACE2 use by different animals may indicate that they are differently susceptible to SARS-CoV-2 infection. Structural and biochemical analysis found that the RBD at the S1-C end of SARS-CoV-2 plays a key role in virus entry and is the target of neutralizing antibodies. Receptor-binding motif (RBM) mediates the contact between the virus and the ACE2 receptor. Biochemical data confirmed that, compared with SARS-CoV, the RBD structural characteristics of SARS-CoV-2 enhanced its binding to hACE2.
Studies have shown that host proteases are involved in the cleavage of S protein and activate the entry of SARS-CoV-2, including transmembrane proteases serine protease 2 (TMPRSS2), cathepsin L and Furin. Single-cell RNA sequencing data showed that TMPRSS2 was highly expressed in multiple tissues and body parts, and co-expressed with ACE2 in nasal epithelial cells, lungs and bronchial branches, which explained part of the tissue tropism of SARS-CoV-2. The analysis of the cryo-electron microscopy structure of SARS-CoV-2 S protein showed that its RBD is mainly in a lysed state. The cleavage conformation of SARS-CoV-2 S protein may not be conducive to receptor binding, but helps immune evasion.
Animal models used to study the pathogenesis of SARS-CoV-2 infection include non-human primates (rhesus monkeys, cynomolgus monkeys, marmosets and African green monkeys), mice, and ferrets. In non-human primate animal models, most species exhibit clinical characteristics similar to those of COVID-19 patients. Animal models provide important information for understanding the pathogenesis of SARS-CoV-2 infection and the transmission dynamics of SARS-CoV-2, and are also important for evaluating the efficacy of antiviral drugs and vaccines.
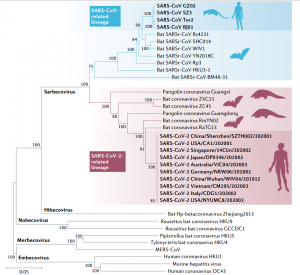
Fig. 2 Phylogenetic tree of the full-length genome sequences of SARS-CoV-2, SARSr-CoVs and other betacoronaviruses.
- Diagnosis, Clinical and Epidemiological Characteristics of COVID-19
Early diagnosis is essential to control the spread of COVID-19. The nucleic acid detection of SARS-CoV-2 is the gold standard. Many viral nucleic acid detection kits for ORF1b (including RdRp), N, E, or S genes have been commercialized, and the detection time ranges from a few minutes to a few hours. Although SARS-CoV-2 has been detected in samples from various respiratory sources, including throat swabs, saliva, nasopharyngeal swabs, sputum, and bronchial fluid, the viral load is higher in lower respiratory tract samples. In addition, there are data showing that even if respiratory samples are negative, viral nucleic acids are found in intestinal or blood samples. Therefore, a variety of testing methods are needed to confirm the diagnosis of COVID-19.
Current data show that people of all ages are susceptible to SARS-CoV-2 infection, and the average age of infection is around 50 years old. Generally speaking, elderly people (60 years old and above) who have other diseases are more likely to develop severe illness or even die. Most young people and children have only mild or asymptomatic symptoms. The risk of pregnant women is not high. However, there is evidence that SARS-CoV-2 may transmit from infected mothers to newborns through the placenta.
The most common symptoms of infection are fever, fatigue and dry cough. Most people develop symptoms within 14 days of the incubation period, and have difficulty breathing and pneumonia within 8 days of onset. The most common patterns seen on chest CT are ground-glass opacity. Most patients also have significant lymphopenia, similar to the situation of SARS and MERS patients. Compared with non-ICU patients, ICU patients have higher plasma cytokine levels, suggesting that there is an immunopathological process caused by cytokine storm.
Since the early SARS-CoV-2 patients found in Wuhan in December 2019 were related to the Huanan Seafood Wholesale Market, the market was once considered to be the source of the outbreak. Later, the emergence of cases unrelated to the market indicated that it was not the source of the virus. The early interpersonal transmission of SARS-CoV-2 in China mostly occurred in family gatherings. In other countries, large-scale epidemics have also occurred in environments such as slaughterhouses and meat processing plants. In some countries, high risks of hospital transmission have also been reported.
The high transmission rate of SARS-CoV-2 may be attributed to the unique virological characteristics of SARS-CoV-2. Studies have confirmed that the risk of virus shedding in the throat of COVID-19 patients is very high when the infection starts. Due to the high transmission rate of the virus in the mild or asymptomatic period, the proportion of asymptomatic infection is high. In addition, airborne transmission also increases the risk of people being infected with SARS-CoV-2. These findings explain the rapid spread of COVID-19 around the world.
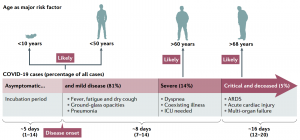
Fig. 3 Clinical features of COVID-19.
- COVID-19 Treatment
So far, there is no universally proven effective COVID-19 therapy or anti-SARS-CoV-2 antiviral drugs. As of October 2, 2020, there are about 405 therapeutic drugs for COVID-19 under development, nearly 318 used in human clinical trials.
- Inhibition of Virus Entry
SARS-CoV-2 uses ACE2 as the receptor and human protease as the activator of entry, and then fuses the viral membrane with the cell membrane to achieve invasion. Therefore, drugs that interfere with invasion may be a potential treatment for COVID-19. Arbidol is a drug approved by Russia and China for the treatment of influenza and other respiratory viral infections. Clinical data shows that it may be effective for the treatment of COVID-19. Camostat mesylate is approved in Japan for the treatment of pancreatitis and postoperative reflux esophagitis. Chloroquine and hydroxychloroquine are used to prevent and treat malaria and autoimmune diseases. Studies have shown that they can inhibit SARS-CoV-2 infection in vitro, but insufficient clinical data may increase the risk of death. On June 15, 2020, the U.S. Food and Drug Administration (FDA) revoked the emergency use license of chloroquine and hydroxychloroquine for the treatment of COVID-19 due to side effects observed in clinical trials. Another possible treatment strategy is to block the binding of S protein to ACE2 through soluble recombinant hACE2 and specific monoclonal antibodies or fusion inhibitors targeting SARS-CoV-2 S protein. The safety and effectiveness of these treatment strategies have yet to be evaluated in future clinical trials.
- Inhibition of Virus Replication
Replication inhibitors include remdesivir (GS-5734), favipiravir (T-705), ribavirin, lopinavir, and ritonavir. Except for lopinavir and ritonavir, which can inhibit 3CLpro, the other three drugs all target RdRp. The FDA has issued an emergency use authorization for Remdesivir for the treatment of hospitalized patients with severe COVID-19. It is also the first option approved by the European Union for the treatment of pneumonia in adults and adolescents that require supplemental oxygen. The antiviral drug Fabiravir (T-705) developed in Japan has been approved by China, Russia and India for the treatment of COVID-19. A clinical study showed that Favipiravir significantly reduced the chest symptoms of patients and shortened the time for virus clearance. In addition, there is no effective evidence to confirm that lopinavir and ritonavir have good clinical performance in the treatment of COVID-19.
- Immunomodulatory Drugs
SARS-CoV-2 triggers a strong immune response. Therefore, immunomodulators that inhibit excessive inflammation may be a potential adjuvant therapy for COVID-19. Recently, data show that dexamethasone reduces the mortality rate of COVID-19 hospitalized patients receiving invasive mechanical ventilation by about one-third, and reduces the mortality rate of patients receiving oxygen therapy by one-fifth. However, there is no obvious benefit to the treatment of patients without respiratory support. Tocilizumab and sarilumab are two interleukin-6 (IL-6) receptor specific antibodies that can treat severe COVID-19 patients by reducing the cytokine storm. Bevacizumab is an anti-vascular endothelial growth factor (VEGF) drug that may reduce pulmonary edema in patients with severe COVID-19. Eculizumab is a specific monoclonal antibody. Current data show that it can reduce inflammation markers and C-reactive protein levels, and may become an option for the treatment of severe COVID-19. Some experiments have shown the potential effectiveness of type I interferon in the early treatment of COVID-19. In China, inhaled interferon-α has been included in the treatment guidelines for COVID-19. Clinical trials are ongoing around the world to evaluate the efficacy of different therapies using interferon alone or in combination with other drugs.
- Immunoglobulin Therapy
Convalescent plasma therapy is another possible adjuvant therapy for COVID-19. The US FDA has provided guidance for the use of COVID-19 convalescent plasma in emergency research applications for new drugs. However, plasma therapy may cause antibody-mediated infection enhancement, blood transfusion-related acute lung injury, and allergic transfusion reactions.
- Vaccines
Vaccines are the most effective method in the long-term strategy of preventing and controlling COVID-19 in the future. Many different anti-SARS-CoV-2 vaccine platforms are under development. Their strategies include recombinant vectors, DNA, mRNA in lipid nanoparticles, inactivated viruses, live attenuated viruses, and protein subunits. As of October 2, 2020, 174 new vaccine candidates for COVID-19 have been reported, and 51 are undergoing human clinical trials.
In Wuhan, a randomized double-blind phase II trial of the SARS-CoV-2 S protein adenovirus type 5 vector vaccine developed by Kang Sino and the Chinese Academy of Military Medical Sciences was conducted on 603 adult volunteers. The vaccine was proved to be safe and induced an immune response in most recipients after a single immunization. Another vector vaccine ChAdOx1 was developed by Oxford University based on chimpanzee adenovirus. In a phase I/II randomized controlled trial, after the second vaccination, ChAdOx1 induced neutralizing antibodies against SARS-CoV-2 in all 1077 participants, and its safety was also recognized. The immunogenicity of mRNA-1273 has been confirmed in a phase I trial. A phase I/II inactivated vaccine trial involving 320 participants was also successfully conducted in China.
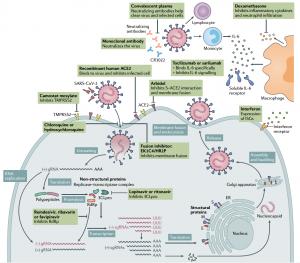
Fig. 4 SARS-CoV-2 replication and potential therapeutic targets.
Although a large number of studies on SARS-CoV-2 are published every week, the current understanding of this new type of coronavirus is only the tip of the iceberg. Although genetic evidence indicates that SARS-CoV-2 is a natural virus that may have originated in animals, there is currently no conclusion on when and where the virus first infected humans.
- What can we do?
Creative Biolabs organized relevant researchers, and set up a dedicated research team to carry out intensive analysis and research on the SARS-CoV-2.
Our team has rapidly developed related proteins, antibodies, nucleic acid extraction kits and other products to help researchers quickly develop effective anti-COVID-19 drugs and vaccines.
We also provide researchers with excellent CRO services regarding to discovery services of anti-SARS-CoV-2 drugs as well as vaccine development services, preclinical research services, various in vivo and in vitro research models, and in vivo diagnostic immunoassay services.
