Background
Recent reports that elevated serum cytokine levels are associated with the 2019 coronavirus (COVID-19) have raised questions about the relationship between cytokine storm and severe complications of this infection. The purpose of this article is to introduce current concepts that contribute to the understanding of the underlying causes of cytokine storms and how they relate to coagulation and vascular health, and to briefly discuss how it can aid in understanding the pathophysiology of COVID-19. Historically, the recognition of sepsis as a clinical condition has been closely associated with the presence of bacterial infection in the blood. This definition has been extended to include all infections or suspected infections that result in immune dysregulation characterized by systemic inflammation and distant organ damage.
Introduction
In June 2020, the research group of Professor Christopher A. Hunter from the University of Pennsylvania School of Veterinary Medicine published an article entitled “Cytokine Storms: Understanding COVID-19” in the journal Immunity (IF: 21.522) [1]. Here, the authors explained the protective function of cytokines in “ideal” responses; the multifactorial origins that can drive these responses to pathology; and how this ultimately leads to vascular damage, immunopathology, and worse clinical outcomes.

Main Results
Cytokine Storm Definition: Basic Concepts of Cytokine Response.
A conceptual framework for how the immune system works is that the inherent ability to recognize invading organisms provides signals that condition the cellular immune system to respond appropriately. This is associated with amplification of protective responses proportional to pathogen load, but is also influenced by regulatory mechanisms that limit immune overactivity. When the infection is under control, there is usually an inflection point associated with entering a phase of decomposition and repair, which allows homeostasis to be restored. Cytokines have a direct role in activating antimicrobial effector functions, but also provide regulatory signals that specify, amplify, and resolve immune responses.

Fig.1 Kinetics of Cytokine Storm
Cytokine storms have many different underlying causes and can exhibit different kinetics. (A) The solid line depicts the natural arc of the immune response over days to weeks after the pathogen has been brought under control. For microorganisms with high replication potential, changes in the magnitude and duration of immune responses can lead to systemic immune pathology. The two dashed lines show distinct arcs of the cytokine storm through increased amplitude or failure to enter the decomposition phase. (B) Rapid and extensive engagement in adaptive responses via bacterial-derived superantigens or therapeutic interventions can lead to a rapid surge in immune activity (hour-days) associated with supraphysiological levels of circulating cytokines. (C) Certain cancers with a systemic component can cause persistent (weeks to months) responses with elevated cytokines. Likewise, chronic autoimmune diseases, such as juvenile idiopathic arthritis (JIA) and lupus erythematosus, are also associated with increased cytokines. There are also genetic defects closely related to abnormal cytokine production, enhanced signaling, or inability to fully control certain viral infections, which lead to periodic immune hyperactivity.
Clinical Impact
Before describing the various events that drive the innate and adaptive responses underlying cytokine storms, it is helpful to consider the clinical consequences of high levels of cytokines. These changes can focus on profound changes in target tissues and host physiology, with no organ spared and host survival threatened. The hallmarks of cytokine storms are persistent fever and nonspecific constitutional symptoms (weight loss, joint and muscle pain, fatigue, headache). Progressive generalized systemic inflammation leads to loss of vascular tone, manifested by decreased blood pressure, vasodilatory shock, and progressive organ failure. Some clinical manifestations are associated with specific cytokines: IL-6 and TNF are associated with fever and constitutional symptoms. Capillary leak syndrome refers to increased capillary permeability to proteins, clinically manifested as hypotension, edema, acute respiratory failure, and renal injury, and is thought to be caused by IL-2. Hepatosplenomegaly predominates in patients with HLH, pancytopenia (penia in red blood cells, white blood cells, and thrombocytopenia), elevated triglycerides, and ferritin. Patients with tumor lysis present with elevated uric acid, lactate dehydrogenase, lactate, and acute kidney injury.
Innate and adaptive immune components contribute to cytokine storm
Increased numbers of monocytes and neutrophils in the bone marrow are responses to many acute infections and cytokines. These cells are generally considered pro-inflammatory, possess a range of antimicrobial activities, and are recruited to sites of inflammation, where they respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by producing IL-1, IL-6, IL-12, and TNF. In many experimental models, such as injection of high doses of lipopolysaccharide (LPS) mimicking gram-negative infection bacterial peritonitis or cecal ligation and perforation models, these innate responses are induced by neutrophils and monocytes to induce a cytokine storm, which bypasses normal immune response kinetics. The primary function of macrophages in the red pulp of the spleen is to remove senescent or damaged red blood cells (erythrophagocytosis), a process that, for some pathogens or red blood cells, is key to clearing infected cells. However, sustained production of IFNγ and TNF can lead to HLH-associated macrophage activation syndrome, leading to anemia characterized by sepsis and nearly all systemic infections.
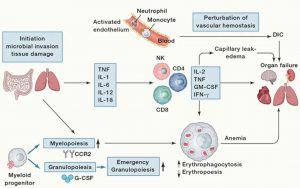
Fig. 2 Pathophysiology of Cytokine Storm
Infectious or non-infectious stimuli at barrier sites, such as the gut or lungs, lead to tissue damage, triggering a complex chain of events. The coagulation system is critical for tissue repair in situations that lead to vascular damage, but this can develop into DIC. The early response to microbial invasion or tissue damage is characterized by the production of cytokines and the induction of emergent granulopoiesis, leading to the mobilization of neutrophils and monocytes. These events will participate in and amplify the production of proinflammatory cytokines by NK and T cells. This can promote capillary leak syndrome and thrombosis, which can lead to the development of DIC. High circulating levels of these cytokines can lead to cell death and tissue damage, while their ability to activate macrophages can lead to erythrophagocytosis and anemia. The combination of anemia, altered vascular hemostasis, and cytokine-mediated damage can lead to multiple organ failures.
COVID-19 and the Cytokine Storm
There is no consensus as to whether COVID-19-related pneumonia and ARDS caused by SARS-CoV-2 are the result of continued viral replication, whether there is abnormal recruitment of macrophages and neutrophils into the lungs, or how the vascular lumen is involved. The kinetics of the response to SARS-CoV-2 are consistent with traditional models of induction of antiviral immunity and a crisis associated with a spike in T-cell responses. However, it’s unclear whether severe disease underlies immune hyperactivity, or an unresolved inflammatory response due to constant viral replication or immune dysregulation. However, reports of elevated levels of thrombosis and endothelial cell death in COVID-19 patients suggest vascular endothelial cell damage, cytokine involvement, and immune thrombosis. This article provides an in-depth review of multiple studies on bioRxiv and medRxiv reporting elevated levels of cytokines and successful use of cytokine antagonists in severe COVID-19 patients.
The presence of elevated levels of cytokines in any patient clinically diagnosed with COVID-19 should not be surprising, but it is unclear whether these levels cross the protective to the pathological threshold. Since existing conditions affecting vascular health, such as diabetes, hypertension, and cardiovascular disease, appear to be the largest single factor in the pathogenesis of COVID-19, these comorbidities may reduce resilience and the ability to tolerate systemic cytokines. Whether patients who survive severe COVID-19 infection experience long-term sequelae similar to those seen in sepsis survivors is still unclear.
Conclusion
The induction and resolution of immune responses have a natural arc, the size and duration of which are coordinated by a series of regulatory checks and balances. Disruption of this balance may result in hyperresponsiveness, or delayed and elevated or sustained cytokine production during the catabolic phase may be pathological. However, not all cytokine storms are created equal, and there are many variables, such as the nature of the injury, the immune status of the host, affected tissues, cross-talk with immune thrombus, complement activation, etc., that influence the magnitude and size of these responses. kinetics, thereby affecting clinical manifestations. However, there are many mechanisms that allow the host to tolerate, survive, and even thrive in the face of immune stress, and understanding these natural processes may help design approaches to manage different forms of CRS. At the time of writing, there is not enough knowledge to confirm that the pathology of COVID-19 is caused by a true cytokine storm, but the rapid progress in the immunophenotype of these patients will help our scientific community understand the disease complexity. Perhaps research into COVID-19 will advance understanding of the consequences of immune dysregulation in the vascular compartment and develop strategies to target cytokines, complement, and coagulation to improve the treatment of other forms of sepsis.
