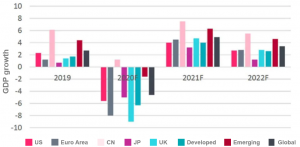
Global Economic Outlook
COVID-19 is tipping the global economy into a tailspin, wiping out years of market gains.
The recession in 2020 is expected to be greater than the 2007-08 crisis:
- Global GDP fell by 4.6%, notably in advanced economies.
- It will bounce back to pre-coronavirus levels by the end of 2021, but the US and eurozone may lag further behind.
Fitch Ratings global Economic Outlook
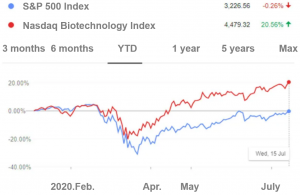
Year-to-date returns, S&P 500 index vs. Nasdaq Biotechnology index
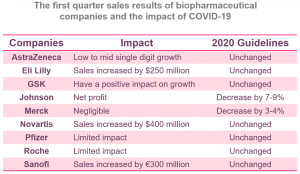
Biopharmaceutical’s First-quarter Earnings Call
While the long-term outlook is unclear, business and underlying demand in the first quarter show some resilience.
Large pharmaceutical companies generally report strong underlying demand and limited impact of COVID-19.
- While one-time treatments are often delayed, sales of long-term Rx and OTC drugs remain robust. Many companies experienced short-term COVID-19 earnings growth due to wholesaler inventories and increased Rx renewals in the first quarter.
- Johnson & Johnson Medical Device Business was exposed for fewer optional procedures.
- Other operations, such as ophthalmology and veterinary medicine, were adversely affected.
While the long-term outlook is unclear, most companies have reaffirmed their previous 2020 guidance.
Business Model During and After COVID-19
Telemedicine provides a different competitive environment for biopharmaceutical companies.
Despite organizational management challenges, the COVID-19 pandemic has made telemedicine a necessity for universal adoption.
- Given its practical benefits, this trend is highly unlikely to reverse.
- Biopharmaceutical companies must adapt to new strategies for doctor-patient interaction and digital sales.
Routine triage of patients during and after COVID-19

Drug properties required for telemedicine practice

Telemedicine will also lead to a realignment of desired target product features.
- Sanofi has raised expectations for Dupixent.
- Is Inclisran still worth $10 billion?
- Interesting case studies of RISdiplam, Spinraza and Zolgensma in the SMA market.
- Developing a telemedicine drug in a clinical trial could give it a competitive labelling advantage.
The transition from Clinical to Commercial
COVID-19 is an important opportunity to rebuild public reputation and trust.
Public opinion polls routinely and consistently paint a very negative picture of pharmaceutical companies.
- According to a 2016 Harris Poll, more than nine out of 10 Americans think drug companies care more about profits than patients.
- The pharmaceutical industry ranked last out of 25 in a Gallup poll released in 2019, below the federal government.
But since the COVID-19 outbreak,Harris has noticed a steady improvement in the perception of the biopharmaceutical industry.
“While the epidemic has devastated many industries, it offers big pharma an unprecedented opportunity to regain the trust of a public angered by years of skyrocketing drug prices. Will they seize the moment?”
The reputational pitfalls that pharmaceutical companies need to deal with are:
- Over-commitment and under-delivery on timetables.
- Manage the transition from clinical to commercial acquisition.
- The rising tide of nationalism against drug/vaccine tenders has lifted all boats.
COVID-19 Presents Many Challenges and Opportunities for the Modernization of the Biopharmaceutical Industry
Clinical research
- Although unprecedented research and development activities are only just beginning to bear fruit, the scale of the collaboration is sure to lead to further success.
- COVID-19 presents the industry with unique challenges, the most pressing of which is the development of clinical trials.
- Enterprise innovation can both sustain operations during the pandemic and modernize, thus achieving long-term benefits.
Regulatory environment
- Regulators act as consultants, authorising biopharmaceutical companies to make judgements about the conduct of clinical trials.
- They also have a range of tools to facilitate and accelerate research into treatments for COVID-19.
- The pace of development of COVID-19 raises questions about whether current research and development models are outdated or even detrimental.
Commercial significance
- Despite the long-term uncertainty posed by the broader macroeconomic situation, valuations and earnings in the biopharmaceuticals industry are relatively well insulated from the specific threat of COVID-19.
- The emergence and consolidation of telemedicine will require adjustment and reprioritization in business practice.
- The industry must regain its credibility and prioritise long-term collective values over short-term individual epidemic gains.
